|
Strain Name
|
C57BL/6-Il6tm1(IL6)BcgenIl6rtm2(IL6R)Bcgen/Bcgen
|
Common Name
|
B-hIL6/hIL6R mice
|
|
Background
|
C57BL/6
|
Catalog number
|
120557
|
Related Genes
|
IL6: CDF; HGF; HSF; BSF2; IL-6; BSF-2; IFNB2; IFN-beta-2
IL6R: IL6Q; gp80; CD126; IL6RA; IL6RQ; IL-6RA; IL-6R-1
|
NCBI Gene ID
|
16193,16194
|
mRNA expression analysis

Strain specific analysis of IL6 and IL6R gene expression in B-hIL6/hIL6R mice by RT-PCR. Mouse Il6 and Il6r mRNA were detectable in spleen of wild-type mice (+/+). Human IL6 was detectable only in homozygous B-hIL6/hIL6R mice (H/H) after stimulated with LPS for 3h. Human IL6R mRNA was detectable in homozygous B-hIL6/hIL6R mice but not in wild-type mice with or without LPS stimulation.
Protein expression analysis
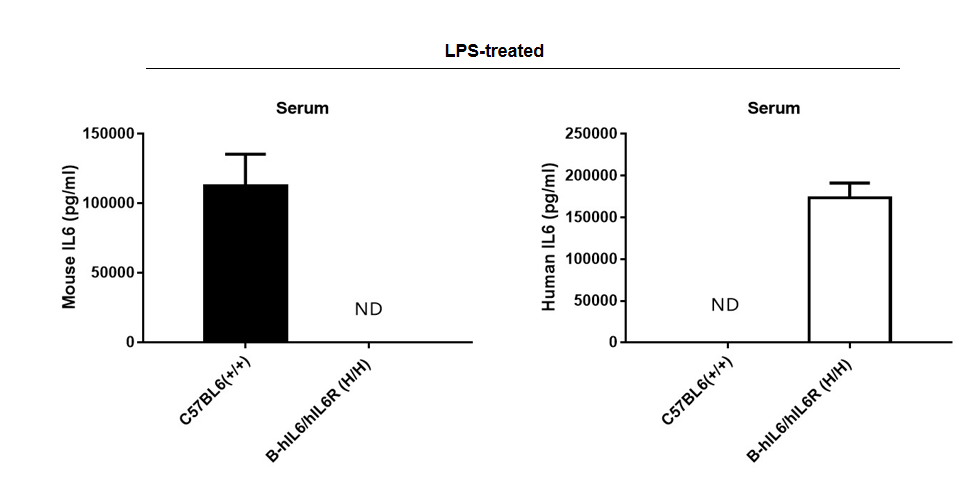
Strain specific IL6 expression analysis in homozygous B-hIL6/hIL6R mice by ELISA.
Serum were collected from WT (+/+) and homozygous B-hIL6/hIL6R (H/H) mice stimulated with LPS in vivo, and analyzed by ELISA with species-specific IL6 ELISA kit. Mouse Il6 was detectable in WT mice. Human IL6 was exclusively detectable in homozygous B-hIL6/hIL6R mice but not in WT mice.
Protein expression analysis
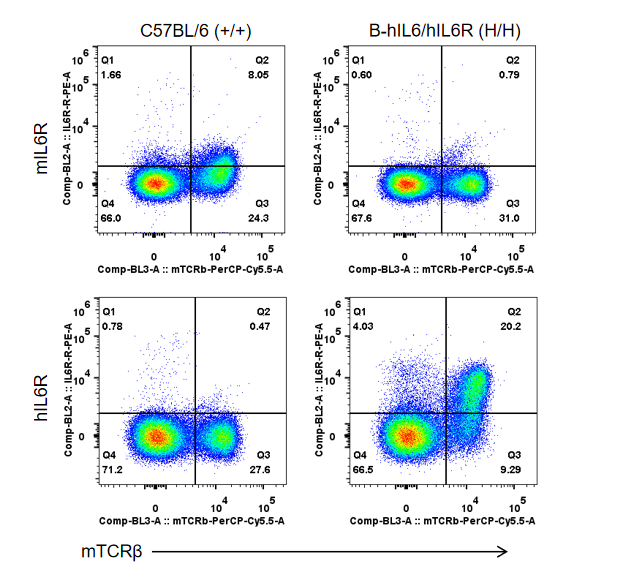
Strain specific IL6R expression analysis in homozygous B-hIL6/hIL6R mice by flow cytometry. Splenocytes were collected from WT (+/+) and homozygous B-hIL6/hIL6R mice (H/H), and analyzed by flow cytometry with species-specific anti-IL6R antibody. Mouse IL6R was detectable in WT mice. Human IL6R was exclusively detectable in homozygous B-hIL6/hIL6R mice but not in WT mice.
IL6R expression pattern analysis by IHC
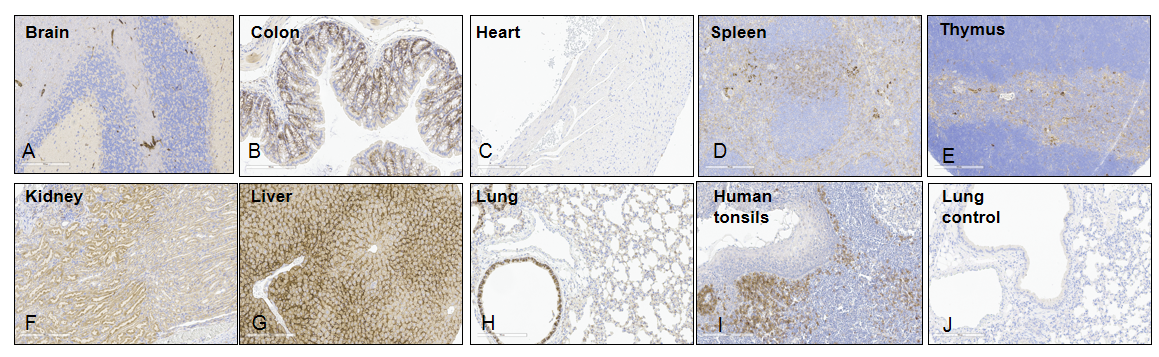
Representative human IL6R expression in different tissues of B-hIL6/IL6R mice by IHC. Tissues were collected from homozygous B-hIL6/hIL6R mice and stained with antibodies for human IL6R (A-I) or anti-IgG antibodies (J). Human tonsils as positive control (I); Mouse lung stained with anti-IgG antibodies as a negative control (J); As shown in the figure, human IL6R was detected in brain, colon, kidney, liver, lung, spleen and thymus, but not in heart. Original magnification ×200. Abbreviations: IHC, immunohistochemistry.
Protein expression analysis
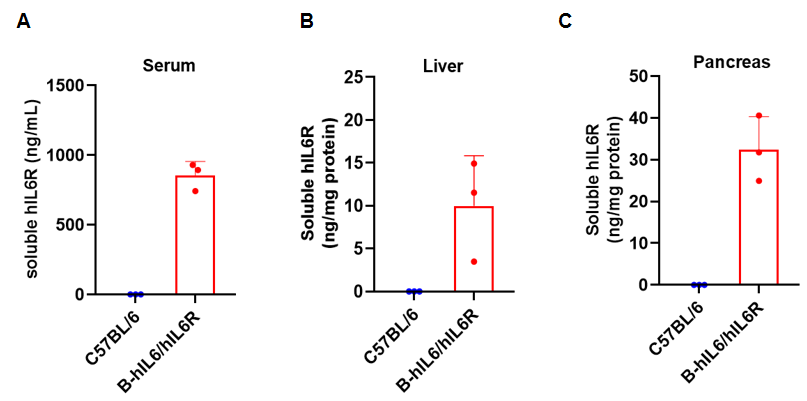
Soluble human IL6R expression analysis in homozygous B-hIL6/hIL6R mice by ELISA. Serum, liver and pancreas were isolated from wild-type C57BL/6 mice and homozygous B-hIL6/hIL6R mice, and analyzed by ELISA with species-specific anti-human IL6R ELISA kit. Soluble human IL6R was detectable in serum (A), liver (B) and pancreas (C) of homozygous B-hIL6/hIL6R mice but not in wild-type mice.
In vivo efficacy of anti-human IL6 antibody
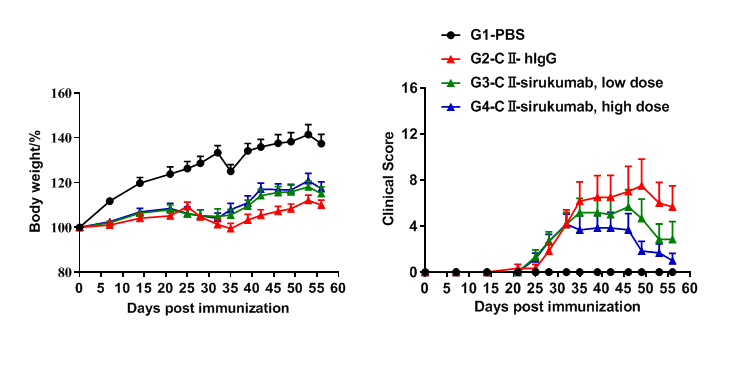
Efficacy of anti-human IL6 antibodies in B-hIL6/hIL6R mice with collagen induced arthritis (CIA) model. Mice in each group were treated with sirukumab (in house). Body weight change(A) and clinical score (B) were evaluated during treatment twice a week. There was no significant change in body weight, while total clinical score increased in the groups except control during days 21 to 28. It indicated that the arthritis mouse model had been constructed successfully. Clinical scores decreased in the two groups treated with sirukumab and dose-dependent. The results indicated the B-hIL6/hIL6R mice provide a powerful preclinical model for in vivo evaluation of anti-human IL6 antibodies.
In vivo efficacy of anti-human IL6 antibody
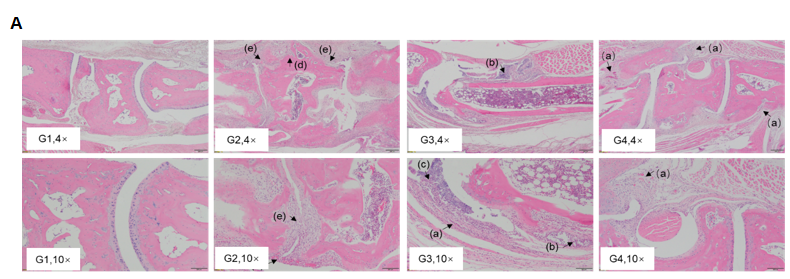
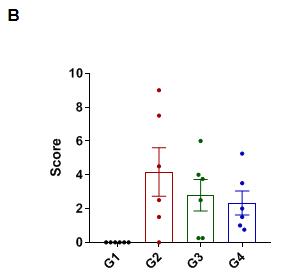
Efficacy of anti-human IL6 antibody in B-hIL6/hIL6R mice with collagen induced arthritis (CIA) model. Histopathological examination was performed on the joints of the extremities at endpoint. (A) Hematoxylin and eosin (H&E) staining. (B) Score of arthritis histology. G1 showed no significant abnormal changes. G2 showed bone structure damage(d), articular cavity or periarticular space disappeared (e), and pannus were observed (a), suggesting that the CIA model was successfully established. Compared with the G2 group, the low-dose group (G3) showed slight inflammatory cell infiltration (b) and pannus (a) and synovial hyperplasia (c). However, in the high dose group (G4), there was only partial pannus, the arthrosis disappeared and the articular cavity was obvious.
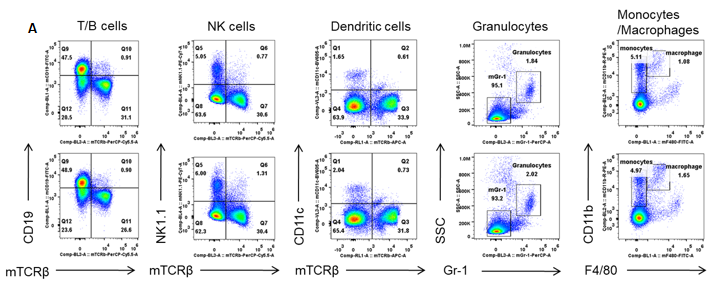
Analysis of spleen leukocytes cell subpopulations in B-hIL6/hIL6R mice
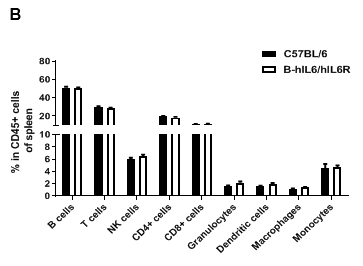
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL6/hIL6R mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes were performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of T cell, B cell, NK cell, monocyte, dendritic cell and macrophage in homozygous B-hIL6/hIL6R mice were similar to those in the C57BL/6 mice, demonstrating that IL6 and IL6R humanized does not change the overall development, differentiation or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
Analysis of spleen T cell subpopulations in B-hIL6/hIL6R mice

Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL6/hIL6R mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes were performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of CD8+ T cell, CD4+ T cell, and Treg in homozygous B-hIL6/hIL6R mice were similar to those in the C57BL/6 mice, demonstrating that IL6 and IL6R humanized does not change the overall development, differentiation or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
Analysis of lymph node leukocytes cell subpopulations in B-hIL6/hIL6R mice
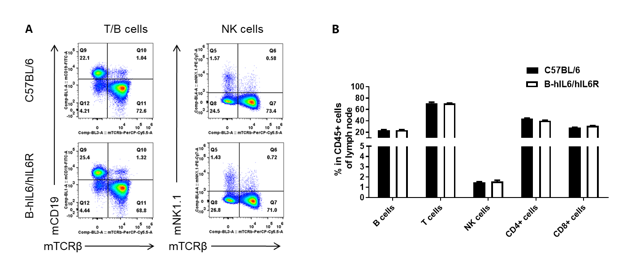
Analysis of lymph node leukocyte subpopulations by FACS. Leukocytes were isolated from female C57BL/6 and B-hIL6/hIL6R mice (n=3, 6-week-old) Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of T cell, B cell and NK cell in homozygous B-hIL6/hIL6R mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL6/hIL6R in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
Analysis of lymph node T cell subpopulations in B-hIL6/hIL6R mice
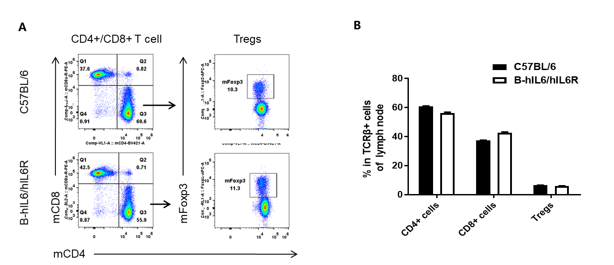
Analysis of lymph node T cell subpopulations by FACS. Leukocytes were isolated from female C57BL/6 and B-hIL6/hIL6R mice (n=3, 6 week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots. Single live CD45+ cells were gated for CD3 T cell population and used for further analysis as indicated here. (B) Results of FACS analysis. Percent of CD8+ cell, CD4+ cell, and Treg in homozygous B-hIL6/hIL6R mice were similar to those in the C57BL/6 mice, demonstrating that IL6 and IL6R humanized does not change the overall development, differentiation or distribution of these T cell subtypes in lymph node. Values are expressed as mean ± SEM.























 京公网安备: 11011502005564号
京公网安备: 11011502005564号